
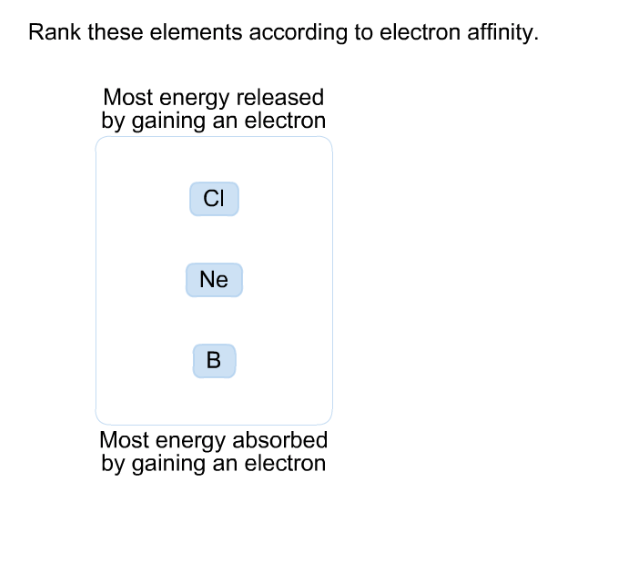
NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties.

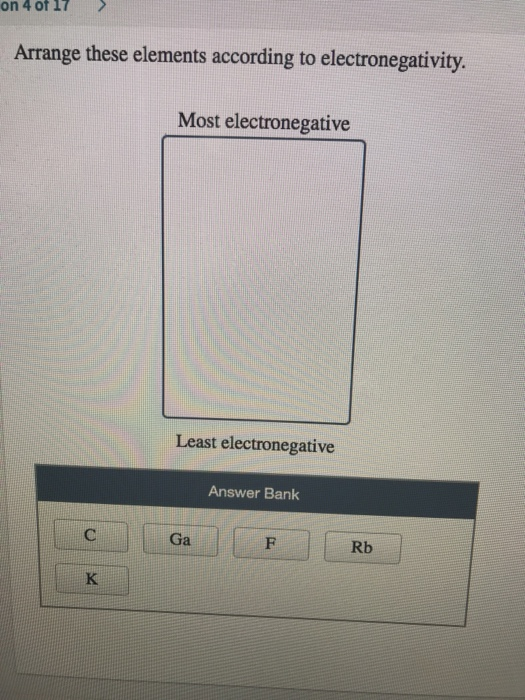
As a result, the value of electronegativity falls as we progress down the group.Įxample: Electronegativity of halogen group decrease as we move down the group from fluorine to astatine and it is shown in the diagram below. The nuclear charge also rises, but the effect is lessened by the addition of one shell. The atomic number grows as we progress down the group on the current periodic chart. Thus, the most electronegativity atom is Chlorine here.Įlectronegativity of elements down a group: Periodic Trends in the Electronegativities of ElementsĮlectronegativity of elements across a period: As we move from left to right through a period in the modern periodic table, the nuclear charge increases and the atomic size decreases, increasing the value of electronegative elements.Įlectronegativity example: Electronegativity trend across period three in the periodic table is shown as.
Arrange these elements according to electronegativity how to#
How to find Electronegativity: Pauling scale Formula of Electronegativity Thus, the element with highest electronegativity is Fluorine. The electrostatic attraction between an atom's nucleus and its valence electrons is measured using the Allred-Rochow scale. Electronegative elements scales include Mulliken scale, which averages first ionisation energy as well as electron affinity. Fluorine has highest electronegativity element on this scale, whereas it is the least electronegative element, with a value of 0.7. Linus Pauling created most often used scale.
As per Pauling scale of electronegativity Fluorine has an electronegativity of 3.98 on the Pauling scale, and the other elements are scaled in relation to it. In 1932, Linus Pauling proposed the idea of electronegative elements. It is represented by the Greek letter chi ‘χ’. It measures how strongly atoms attract bonding electrons to themselves. As you can see, electronegativities generally increase from left to right across a period and decrease down a group.Electronegativity definition as the measure of ability of an atom or atoms involved in covalent bonding s to attract shared electrons to itself. A value of 4.0 is assigned to fluorine, the most electronegative element. The periodic table below shows the Pauling electronegativity scale. Than the other atom, the electrons will not be shared and an ionic bond will If one atom is overwhelmingly more electronegative If one atom is more electronegative, the electrons of the bond are Two atoms of the bond are of equal electronegativity, the electrons are equally Team is no longer able to hold onto the rope and the entire rope ends up on If one team is overwhelmingly stronger, the weaker If one team is stronger, the rope is pulled Will not be shared at all the more electronegative atom will "take" them resulting If the difference in electronegativity is large enough, the electrons Of the atoms (because it is more electronegative), the electrons will be unequally If the electrons of a bond are more attracted to one If atoms bonded together have the same electronegativity, the shared electrons An atom's ability to attract the shared electrons of a covalent bond to itself.


 0 kommentar(er)
0 kommentar(er)
